What are Acid Dyes?
Acid dyes are a type of water-soluble dyes with acidic groups in their structure, which are dyed in acidic media. Most acid dyes contain sodium sulfonate, which is soluble in water and has bright colors and complete chromatograms. Acid dyes can combine with amino or amide groups on fibers, so they can be used for dyeing and printing of protein fibers such as wool and silk, and polyamide synthetic fibers. Acid dyes have bright colors and good dye fastness. Most of these dyes are dyed in acidic solutions, so they are called acid dye dyeing.

Compared with direct dyes, acid dyes have a simple structure and lack long conjugated double bonds and a co-planar structure. Therefore, they lack directness to cellulose fibers and cannot be used for dyeing cellulose fibers. Different types of acid dyes have different dyeing properties due to different molecular structures, and the dyeing methods used are also different. The light fastness and wet treatment fastness of acid dyes vary greatly with different dye varieties.
The Science behind Acid Dyes
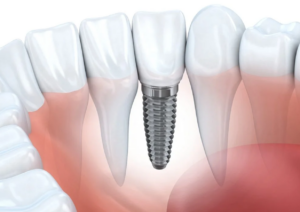
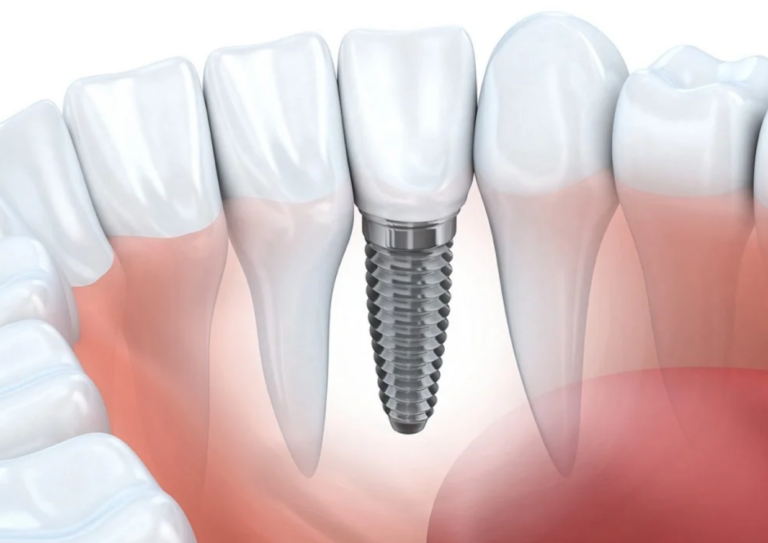
Acid dyes are mainly used to dye protein fibers and nylon. Wool, silk, and polyamide fibers contain amino and carboxyl groups H2N—W—COOH. When the fiber enters the aqueous solution, the amino and carboxyl groups dissociate in the water to form zwitterionic + H3N—W—COO–. The pH value of the isoelectric point of wool is 4.2~4.8. When the pH value of the solution is greater than the isoelectric point, the fiber surface is negatively charged; when the solution pH value is small and the isoelectric point of the fiber, the fiber surface is positively charged.

Acid dyes are ionized in the dye bath. In the strong acid dye dye bath, the fiber surface is positively charged, the dye ions are negatively charged, and the fibers and dyes are combined through ionic bonds; in weakly acidic baths and neutral baths, the fiber surface is negatively charged, and the dye ions are also negatively charged. At this time, the fiber and dye are combined through van der Waals forces and hydrogen bonds.
Types of Acid Dyes
According to the classification of chemical structure, acid dyes can be divided into azo, anthraquinone, triarylmethane and other categories.
①Azo type: Mono- and disazo dyes, mainly light colors (yellow, orange, red, purple, blue), accounting for the majority of varieties;
②Anthraquinone type: monoanthraquinone is the main type, mainly dark-colored (purple, blue, green) varieties occupy the second place, and have the best light fastness
③Triaromatic methane type: bright color, high strength, mainly purple, blue and green, with the worst light fastness.
Strong acid dye: The earliest developed acid dye, which requires dyeing in a strong acid dye bath. Its molecular structure is simple, its molecular weight is low, it contains sulfonic acid groups or carboxyl groups, and the sulfonic acid group accounts for a large proportion in the molecule. It also has strong hydrophilicity, weak hydrophobicity, good water solubility, and low affinity for wool. It can transfer dye to wool and dye it evenly, but it will damage the wool during dyeing and the wool after dyeing will have a poor feel. It needs to be dyed in a strong acid bath to obtain a high dye uptake rate, bright color, suitable for dyeing light and medium colors, and is mainly used for wool dyeing.
Weak acid dyes: Weak acid dyes can be generated from strong acid dyes by increasing the molecular weight, introducing groups such as aryl sulfone groups or introducing long carbon chains. The molecular structure is complex, the proportion of sulfonic acid groups in the molecule is relatively small, the hydrophilicity, aggregation degree and solubility in aqueous solutions are moderate, and it has a high affinity for wool and can dye wool in weakly acidic media.
Acid mordant dyes: Acid dyes that form metal complexes on fabrics after treatment with certain metal salts (such as chromium salts, copper salts, etc.). The procedures for mordant dyeing are complicated, but the properties such as light fastness, washing fastness and rubbing fastness are better.
Acid complex dyes: composed of certain acid dyes complexed with metals such as chromium and cobalt. It is soluble in water and has excellent light fastness and light fastness. There is no need to use mordant when dyeing, and it must be dyed in a strong acid bath.